Share this
Authors
Shohei Tada, Fumito Otsuka, Kakeru Fujiwara, Constantinos Moularas, Yiannis Deligiannakis, Yuki Kinoshita, Sayaka Uchida, Tetsuo Honma, Masahiko Nishijima, Ryuji Kikuchi
Abstract
Dispersion of metallic Cu nanoparticles on a metal oxide support increases the number of exposed metallic Cu sites and/or Cu-support interfacial sites, resulting in good catalytic performance for CO2-to-methanol hydrogenation. However, the formation of highly dispersed Cu nanoparticles is challenging because they are easily sintered. Here, we studied Cu nanoparticle formation by a simple deposition–reduction technique using Cu-doped MgAl2O4 (Mg1–xCuxAl2O4). Mg1–xCuxAl2O4 possessed the following three types of Cu2+ species: short O–Cu octahedrally coordinated [CuO6]s, elongated O–Cu octahedrally coordinated [CuO6]el, and tetrahedrally coordinated [CuO4]t. The former two are found in the inverse-spinel-type Mg1–xCuxAl2O4, while the other is found in the normal-spinel-type Mg1–xCuxAl2O4. Additionally, by focusing on the difference in the reducibility of the Cu2+ species, we clarified that their fraction is related to Cu loading. For low Cu loading (x < 0.3), Mg1–xCuxAl2O4 mainly contained the [CuO6]s species. On the other hand, for high Cu loading (x ≥ 0.3), the fraction of the [CuO6]el and [CuO4]t species increased. Notably, among the prepared catalysts, H2-reduced Mg0.8Cu0.2Al2O4 (x = 0.2) had the largest number of exposed metallic Cu sites, resulting in its good catalytic performance. Hence, the H2 reduction of [CuO6]s is essential for forming metallic Cu nanoparticles on metal oxides.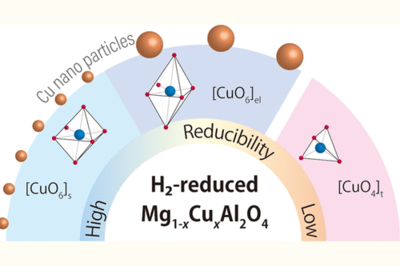
ACS Catalysis : https://pubs.acs.org/doi/10.1021/acscatal.0c02868
These Related Stories