Share this
Authors
Morito Sakuma, Shingo Honda, Hiroshi Ueno, Kazuhito V. Tabata, Kentaro Miyazaki, Nobuhiko Tokuriki and Hiroyuki Noji
Abstract
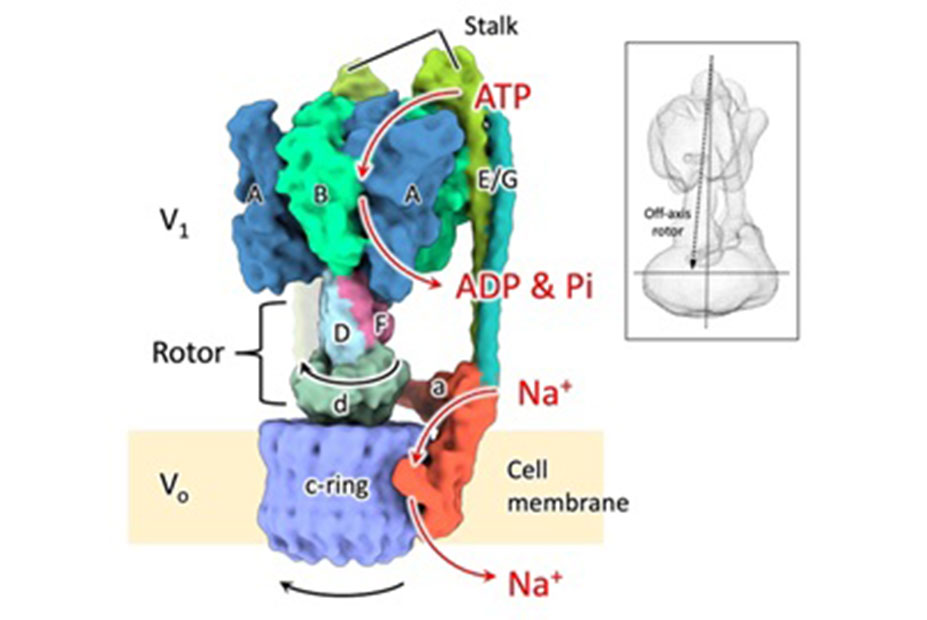
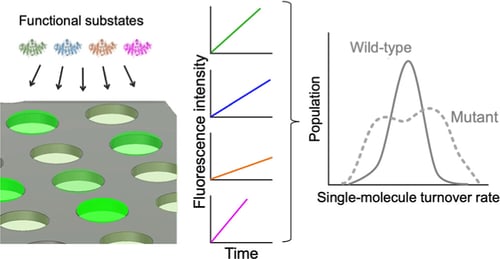
Enzymes inherently exhibit molecule-to-molecule heterogeneity in their conformational and functional states, which is considered to be a key to the evolution of new functions. Single-molecule enzyme assays enable us to directly observe such multiple functional states or functional substates. Here, we quantitatively analyzed functional substates in the wild-type and 69 single-point mutants of Escherichia coli alkaline phosphatase by employing a high-throughput single-molecule assay with a femtoliter reactor array device. Interestingly, many mutant enzymes exhibited significantly heterogeneous functional substates with various types, while the wild-type enzyme showed a highly homogeneous substate. We identified a correlation between the degree of functional substates and the level of improvement in promiscuous activities. Our work provides much comprehensive evidence that the functional substates can be easily altered by mutations, and the evolution toward a new catalytic activity may involve the modulation of the functional substates.
Journal of the American Chemical Society: https://pubs.acs.org/doi/10.1021/jacs.2c06693
These Related Stories