Share this
Authors
P.Chen, W.Yang, K.Nagaoka, G.L.Huang, T.Miyazaki, T.Hong, S.Li, K.Igarashi, K.Takeda, K.Kakimi, K.Kataoka and H.Cabral
Abstract
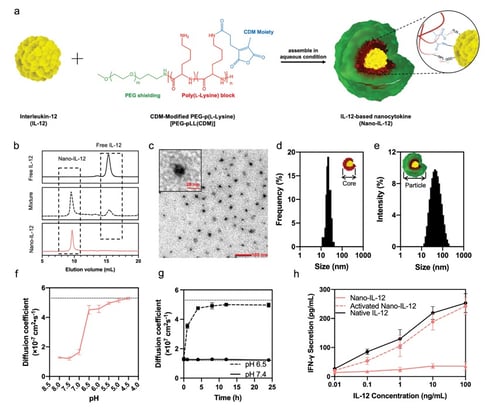
Treatment of immunologically cold tumors is a major challenge for immune checkpoint inhibitors (ICIs). Interleukin 12 (IL-12) can invigorate ICIs against cold tumors by establishing a robust antitumor immunity. However, its toxicity and systemic induction of counteracting immunosuppressive signals have hindered translation. Here, IL-12 activity is spatiotemporally controlled for safely boosting efficacy without the stimulation of interfering immune responses by generating a nanocytokine that remains inactive at physiological pH, but unleashes its full activity at acidic tumor pH. The IL-12-based nanocytokine (Nano-IL-12) accumulate and release IL-12 in tumor tissues, eliciting localized antitumoral inflammation, while preventing systemic immune response, counteractive immune reactions, and adverse toxicities even after repeated intravenous administration. The Nano-IL-12-mediated spatiotemporal control of inflammation prompt superior anticancer efficacy, and synergize with ICIs to profoundly inflame the tumor microenvironment and completely eradicate ICI-resistant primary and metastatic tumors. The strategy could be a promising approach toward safer and more effective immunotherapies.
Advanced Science: https://onlinelibrary.wiley.com/doi/10.1002/advs.202205139
These Related Stories