Share this
Authors
Takafumi Yatabe, Kazuya Yamaguchi
Abstract
Regioselective transformations of tertiary amines, which are ubiquitously present in natural products and drugs, are important for the development of novel medicines. In particular, the oxidative α-C–H functionalisation of tertiary amines with nucleophiles via iminium cations is a promising approach because, theoretically, there is almost no limit to the type of amine and functionalisation. However, most of the reports on oxidative α-C–H functionalisations are limited to α-methyl-selective or non-selective reactions, despite the frequent appearance of α-methylene-substituted amines in pharmaceutical fields. Herein, we develop an unusual oxidative regiospecific α-methylene functionalisation of structurally diverse tertiary amines with alkynes to synthesise various propargylic amines using a catalyst comprising Zn salts and hydroxyapatite-supported Au nanoparticles. Thorough experimental investigations suggest that the unusual α-methylene regiospecificity is probably due to a concerted one-proton/two-electron transfer from amines to O2 on the Au nanoparticle catalyst, which paves the way to other α-methylene-specific functionalisations.
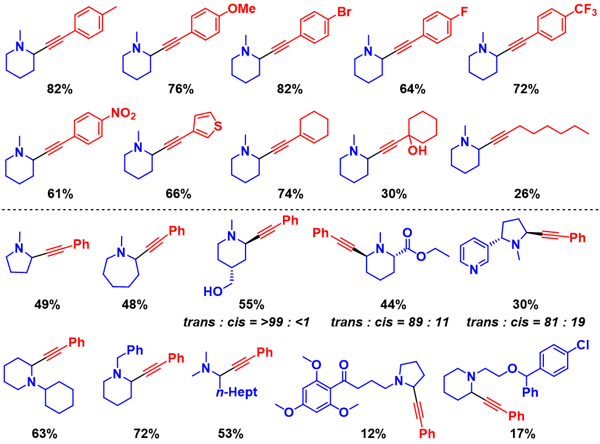
Nature Communications: https://www.nature.com/articles/s41467-022-34176-x
These Related Stories