Share this
Authors
Takefumi Yamashita, Eiichi Mizohata, Satoru Nagatoishi, Takahiro Watanabe, Makoto Nakakido, Hiroko Iwanari, Yasuhiro Mochizuki, Taisuke Nakayama, Yuji Kado, Yuki Yokota, Hiroyoshi Matsumura, Takeshi Kawamura, Tatsuhiko Kodama, Takao Hamakubo, Tsuyoshi Inoue, Hideaki Fujitani, and Kouhei Tsumoto
Abstract
To investigate favorable single amino acid substitutions that improve antigen-antibody interactions, alanine (Ala) mutagenesis scanning of the interfacial residues of a cancer-targeted antibody, B5209B, was performed based on X-ray crystallography analysis. Two substitutions were shown to significantly enhance the binding affinity for the antigen, by up to 30-fold. One substitution improved the affinity by a gain of binding enthalpy, whereas the other substitution improved the affinity by a gain of binding entropy. Molecular dynamics simulations showed that the enthalpic improvement could be attributed to the stabilization of distant salt bridges located at the periphery of the antigen-antibody interface. The entropic improvement was due to the release of water molecules that were stably trapped in the antigen-antibody interface of the wild-type antibody. Importantly, these effects of the Ala substitutions were caused by subtle adjustments of the binding interface. These results will be helpful to design high-affinity antibodies with avoiding entropy-enthalpy compensation.
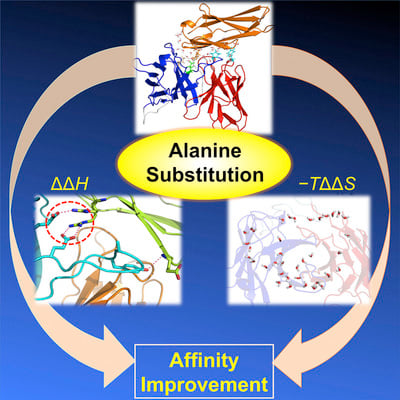
Structure : https://doi.org/10.1016/j.str.2018.11.002
These Related Stories