Share this
Authors
Yulin Zhang, Yoshiaki Tanabe, Shogo Kuriyama, Ken Sakata and Yoshiaki Nishibayashi
Abstract
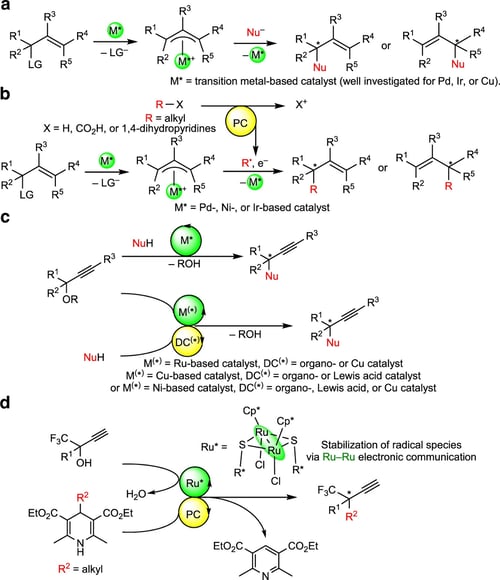
Transition metal-catalyzed enantioselective free radical substitution reactions have recently attracted attention as convenient and important building tools in synthetic chemistry, although construction of stereogenic carbon centers at the propargylic position of propargylic alcohols by reactions with free radicals remains unchallenged. Here we present a strategy to control enantioselective propargylic substitution reactions with alkyl radicals under photoredox conditions by applying dual photoredox and diruthenium catalytic system, where the photoredox catalyst generates alkyl radicals from 4-alkyl-1,4-dihydropyridines, and the diruthenium core with a chiral ligand traps propargylic alcohols and alkyl radicals to guide enantioselective alkylation at the propargylic position, leading to high yields of propargylic alkylated products containing a quaternary stereogenic carbon center at the propargylic position with a high enantioselectivity. The result described in this paper provides the successful example of transition metal-catalyzed enantioselective propargylic substitution reactions with free alkyl radicals.
Nature Communications: https://www.nature.com/articles/s41467-023-36453-9
These Related Stories