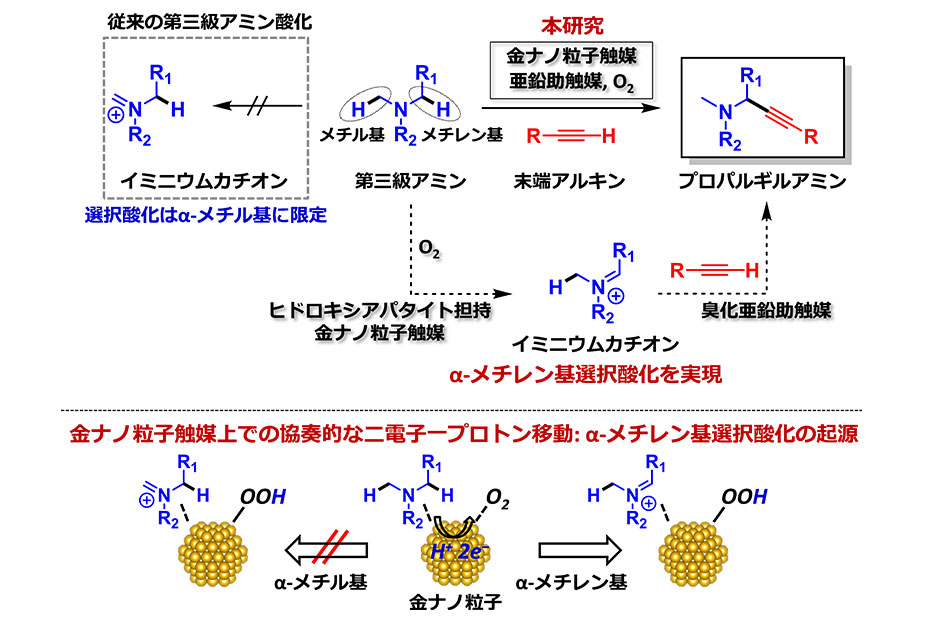
Share this
Authors
Yuta Noda, Shunpei Okada and Tsutomu Suzuki
Abstract
Selenoprotein N (SELENON), a selenocysteine (Sec)-containing protein with high reductive activity, maintains redox homeostasis, thereby contributing to skeletal muscle differentiation and function. Loss-of-function mutations in SELENON cause severe neuromuscular disorders. In the early-to-middle stage of myoblast differentiation, SELENON maintains redox homeostasis and modulates endoplasmic reticulum (ER) Ca2+ concentration, resulting in a gradual reduction from the middle-to-late stages due to unknown mechanisms. The present study describes post-transcriptional mechanisms that regulate SELENON expression during myoblast differentiation. Part of an Alu element in the second intron of SELENON pre-mRNA is frequently exonized during splicing, resulting in an aberrant mRNA that is degraded by nonsense-mediated mRNA decay (NMD). In the middle stage of myoblast differentiation, ADAR1-mediated A-to-I RNA editing occurs in the U1 snRNA binding site at 5′ splice site, preventing Alu exonization and producing mature mRNA. In the middle-to-late stage of myoblast differentiation, the level of Sec-charged tRNASec decreases due to downregulation of essential recoding factors for Sec insertion, thereby generating a premature termination codon in SELENON mRNA, which is targeted by NMD.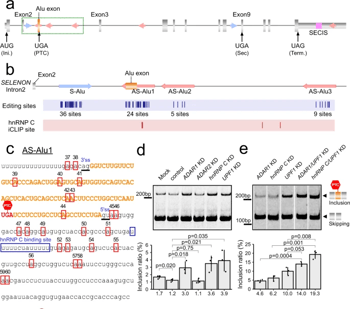
Nature Communications: https://www.nature.com/articles/s41467-022-30181-2
These Related Stories