Share this
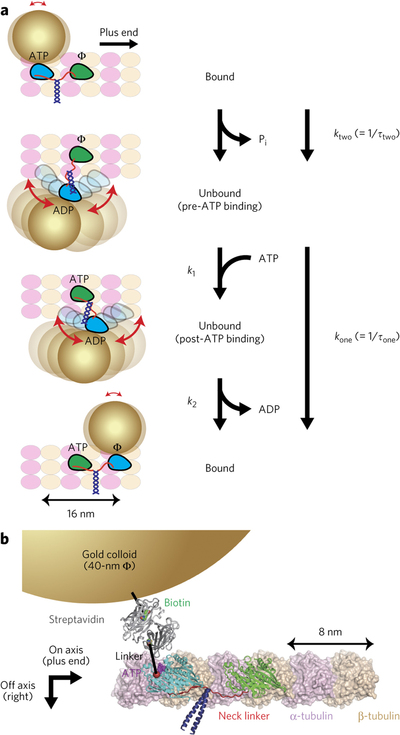
The dimeric motor protein kinesin-1 walks along microtubules by alternatingly hydrolyzing ATP and moving two motor domains ('heads'). Nanometer-precision single-molecule studies demonstrated that kinesin takes regular 8-nm steps upon hydrolysis of each ATP; however, the intermediate states between steps have not been directly visualized. Here, we employed high-temporal resolution dark-field microscopy to directly visualize the binding and unbinding of kinesin heads to or from microtubules during processive movement. Our observations revealed that upon unbinding from microtubules, the labeled heads were displaced rightward and underwent tethered diffusive movement. Structural and kinetic analyses of wild-type and mutant kinesins with altered neck linker lengths provided evidence that rebinding of the unbound head to the rear-binding site is prohibited by a tension increase in the neck linker and that ATP hydrolysis by the leading head is suppressed when both heads are bound to the microtubule, thereby explaining how the two heads coordinate to move in a hand-over-hand manner.
Abstract URL:https://www.nature.com/articles/nchembio.2028
These Related Stories