Share this
Authors
Jinbing Xie, Daniel Gonzalez-Carter, Theofilus A. Tockary, Noriko Nakamura, Yonger Xue, Makoto Nakakido, Hiroki Akiba, Anjaneyulu Dirisala, Xueying Liu, Kazuko Toh, Tao Yang, Zengtao Wang, Shigeto Fukushima, Junjie Li, Sabina Quader, Kouhei Tsumoto, Takanori Yokota, Yasutaka Anraku, and Kazunori Kataoka
Abstract
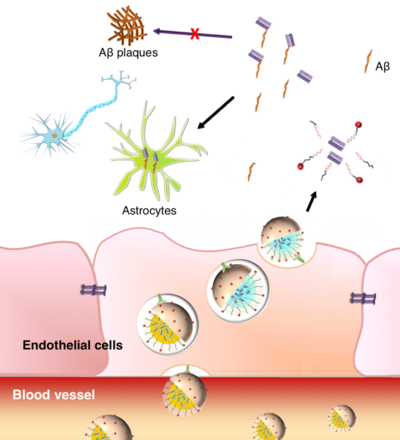
Delivering therapeutic antibodies into the brain across the blood–brain barrier at a therapeutic level is a promising while challenging approach in the treatment of neurological disorders. Here, we present a polymeric nanomicelle (PM) system capable of delivering therapeutically effective levels of 3D6 antibody fragments (3D6-Fab) into the brain parenchyma for inhibiting Aβ aggregation. PM assembly was achieved by charge-converting 3D6-Fab through pH-sensitive citraconylation to allow complexation with reductive-sensitive cationic polymers. Brain targeting was achieved by functionalizing the PM surface with glucose molecules to allow interaction with recycling glucose transporter (Glut)-1 proteins. Consequently, 41-fold enhanced 3D6-Fab accumulation in the brain was achieved by using the PM system compared to free 3D6-Fab. Furthermore, therapeutic benefits were obtained by successfully inhibiting Aβ1–42 aggregation in Alzheimer’s disease mice systemically treated with 3D6-Fab-loaded glucosylated PM. Hence, this nanocarrier system represents a promising method for effectively delivering functional antibody agents into the brain and treating neurological diseases.
These Related Stories