Share this
Authors
Panagi, F. Mpekris, P. Chen, C. Voutouri, Y. Nakagawa, J. D. Martin, T. Hiroi, H. Hashimoto, P. Demetriou, C. Pierides, R. Samuel, A. Stylianou, C. Michael, S. Fukushima, P. Georgiou, P. Papageorgis, P. C. Papaphilippou, L. Koumas, P. Costeas, G. Ishii, M. Kojima, K. Kataoka, H. Cabral and T. Stylianopoulos
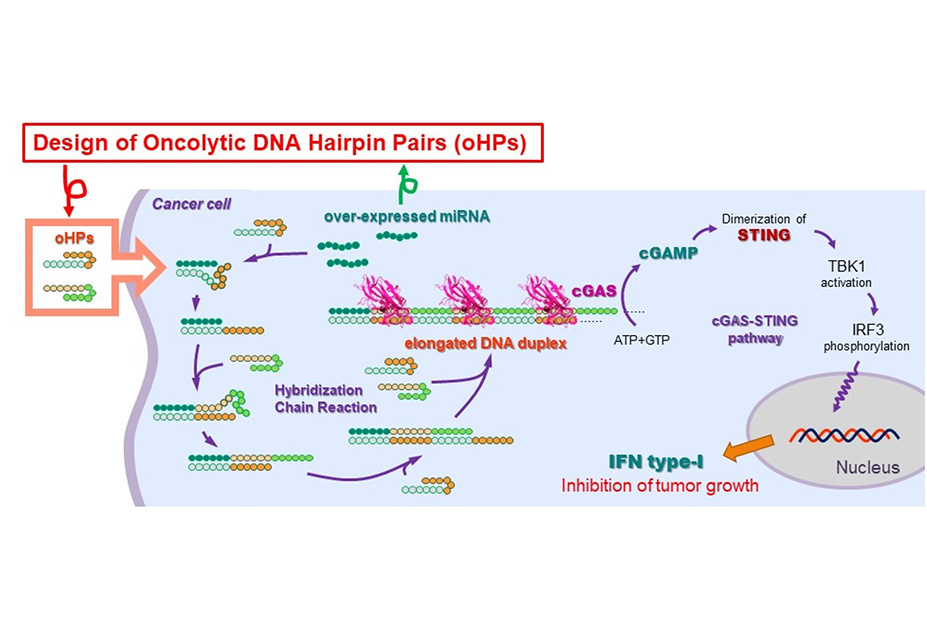
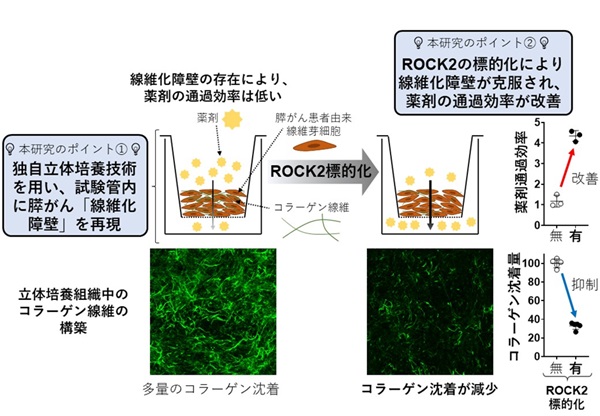
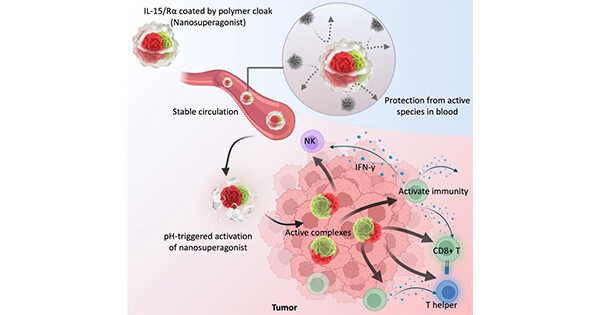
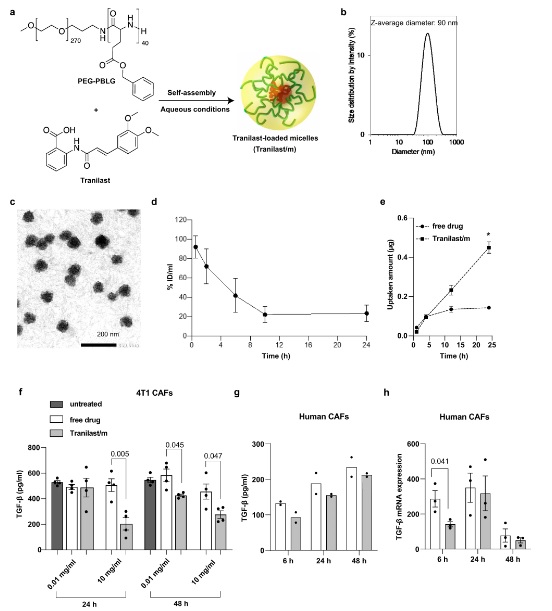
Nano-immunotherapy improves breast cancer outcomes but not all patients respond and none are cured. To improve efficacy, research focuses on drugs that reprogram cancer-associated fibroblasts (CAFs) to improve therapeutic delivery and immunostimulation. These drugs, however, have a narrow therapeutic window and cause adverse effects. Developing strategies that increase CAF-reprogramming while limiting adverse effects is urgent. Here, taking advantage of the CAF-reprogramming capabilities of tranilast, we developed tranilast-loaded micelles. Strikingly, a 100-fold reduced dose of tranilast-micelles induces superior reprogramming compared to free drug owing to enhanced intratumoral accumulation and cancer-associated fibroblast uptake. Combination of tranilast-micelles and epirubicin-micelles or Doxil with immunotherapy increases T-cell infiltration, resulting in cures and immunological memory in mice bearing immunotherapy-resistant breast cancer. Furthermore, shear wave elastography (SWE) is able to monitor reduced tumor stiffness caused by tranilast-micelles and predict response to nano-immunotherapy. Micellar encapsulation is a promising strategy for TME-reprogramming and SWE is a potential biomarker of response.
Nature Communications: https://www.nature.com/articles/s41467-022-34744-1
These Related Stories