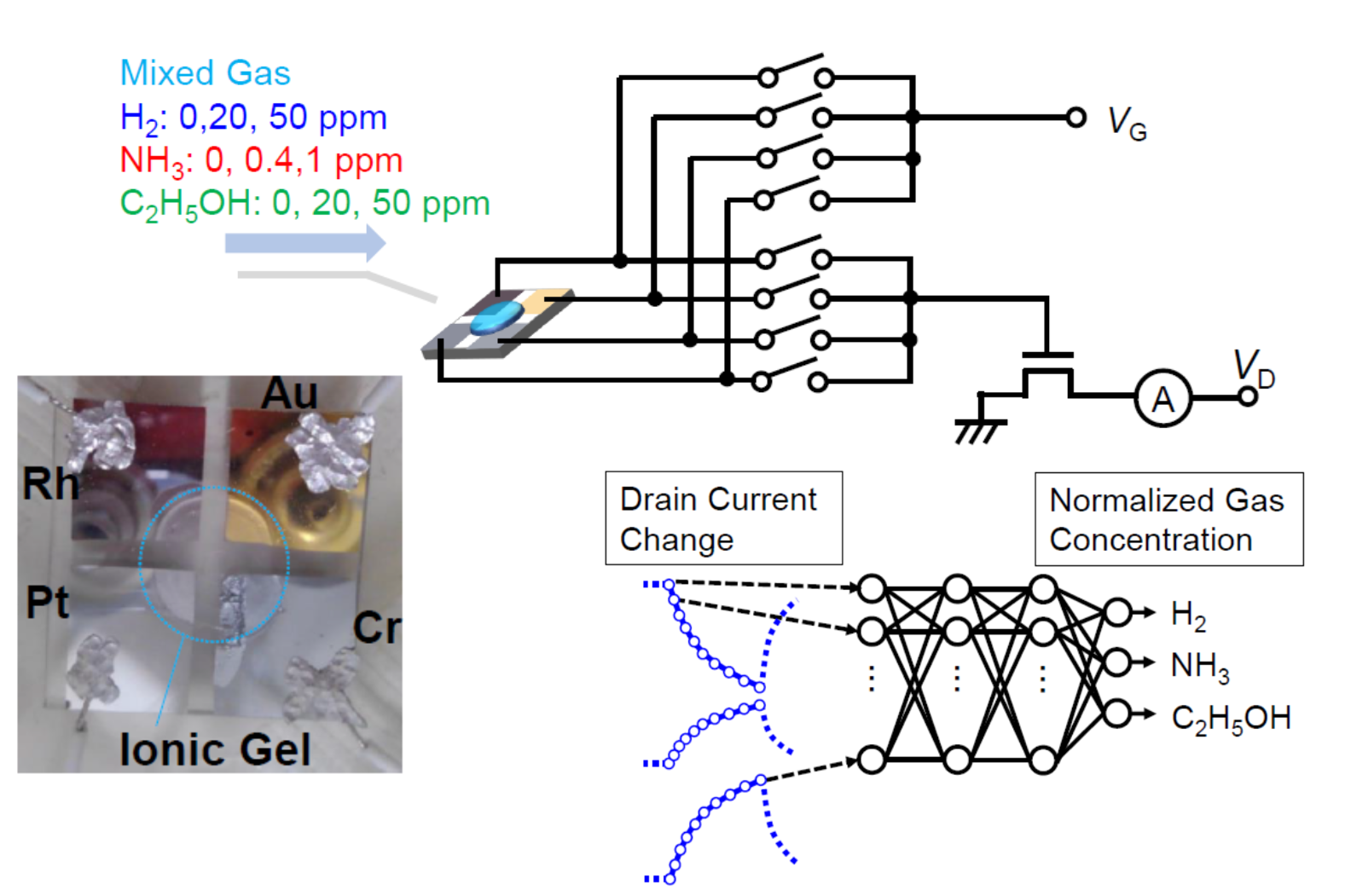
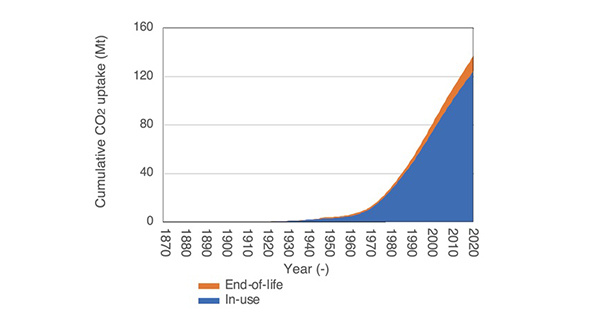
Share this
Ammonia (NH3) has recently attracted attention as an environmentally friendly energy carrier that does not emit CO2 during combustion. However, the current NH3 production method from N2 and H2 (the Haber-Bosch process) requires harsh reaction conditions and is accompanied by much fossil fuel consumption. As an alternative method, Prof. Nishibayashi’s group has developed catalytic reactions to synthesize NH3 from N2 and H2O under ambient conditions using molybdenum complexes as catalysts in solution. These methods enable NH3 production under very mild conditions; however, the use of large amounts of organic solvent is one of the obstacles to the practical application. This study demonstrates NH3 synthesis from N2 under mild conditions without using any organic solvent in the presence of molybdenum catalysts by adopting a ball-milling technique. This is the first solid-state NH3 synthesis using molecular catalysts. In addition, cellulose, an insoluble compound that does not react in solution conditions using organic solvents, can be used as a proton source for NH3 synthesis. Mechanochemical ammonia synthesis achieved in this study is an essential step toward the practical application of nitrogen fixation using molecular catalysts.

Papers
Journal: Nature Synthesis
Title: Mechanochemical nitrogen fixation catalysed by molybdenum complexes
Authors: Shun Suginome, Kurumi Murota, Akira Yamamoto, Hisao Yoshida, and Yoshiaki Nishibayashi*
These Related Stories