Mathematical model development of cultivation process with full utilization of data from automated experiments
Graduate School of Engineering, The University of Tokyo
Chitose Laboratory Corporation
Key Points of Presentation
-
Based on the data obtained from automated experiments on the cultivation process in monoclonal antibody manufacturing, we have developed a new mathematical model, which can accurately predict the concentrations of target products and impurities under a wide range of cultivation conditions.
-
The developed mathematical model has enabled the identification of the “design space” for cultivation conditions that meet control limits for impurities as well as the optimal conditions for maximizing antibody concentrations using simulations.
-
The results of this study will pave the way for process design using digital technologies through the integration of automated experiments and mathematical modeling and will contribute to the acceleration of drug process development and the rapid launch of new drugs.

Digital design through the integration of automated experiments and mathematical modeling
Overview
A research group of Kosuke Nemoto (Ph.D Student), Yuki Yoshiyama (Ph.D Student), Yusuke Hayashi (Assistant Professor), Sara Badr (Associate Professor), and Hirokazu Sugiyama (Professor) in the Department of Chemical System Engineering, Graduate School of Engineering, The University of Tokyo, and Mizuki Morisasa (Researcher) and Junshin Iwabuchi (Researcher) at Chitose Laboratory Corporation, the core company of CHITOSE Group, has developed a new mathematical model, which can accurately predict the concentrations of target product antibody and multiple impurities under a wide range of cultivation conditions in the cultivation process in the manufacture of monoclonal antibodies*1 (mAbs). This model has demonstrated to enable the identification of optimal cultivation conditions using simulation.
mAbs, which play a crucial role in the treatment of serious diseases such as cancer and Alzheimer’s disease, are manufactured through cultivating animal cells to produce the antibody and its separation and purification. In the cultivation process, there are many design variables, but it is difficult to develop a mathematical model that can handle them simultaneously, which has been a barrier to simulation design. In this study, we developed a mathematical model that can describe the concentrations of not only mAb, but also multiple impurities based on the experimental data obtained by automatic cultivation equipment and experimentally confirmed the predictive accuracy. The developed model has enabled the identification of the design space*2 for cultivation conditions that meet control limits for impurities as well as the optimal conditions for maximizing antibody concentrations using simulations. This achievement will contribute to the acceleration of drug process development through digital technologies. The study utilized small-scale, parallelized automated cultivation equipment and demonstrated the usefulness of an approach to integrating automated experiments with mathematical modeling.
The results of the present study were published on-line in AIChE Journal (open access) on February 12, 2026 (Japan Standard Time).
Presentation Details
(1) Background
Monoclonal antibodies (mAbs) are representative active ingredients in biopharmaceuticals and are essential in the treatment of serious diseases such as cancer and Alzheimer’s disease, and their market has been expanding rapidly in recent years. mAbs are manufactured first through cultivating animal cells to produce the antibody during the cultivation process (Figure 1), followed by the antibody separation and purification steps. The cultivation process produces multiple impurities in addition to the intended product mAb, and these impurities are removed in the subsequent purification step. Since the purification process is costly, it is essential to maximize mAb concentration while keeping impurity concentrations low in the cultivation process.

Figure 1 Cultivation process and schematic of the main phenomena (Excerpt from [Nemoto, et al., 2026]; Used with permission)
mAb is the main product, and host cell proteins (HCP)*3, DNA, and charge variants*4 are impurities.
In the pharmaceutical industry, the concept of Quality by Design (QbD)*5 is recommended to meet quality requirements from the design stage. As a means of achieving this, utilization of mathematical models is gaining attention to move away from trial-and-error experiments, and in recent years, especially mathematical models of the cultivation process have been actively studied. However, it is extremely difficult to develop a mathematical model that can be applied to a wide range of cultivation conditions, taking into account multiple impurities for a cultivation process in which countless biological phenomena occur simultaneously. This difficulty has been a major barrier to designing using mathematical models.
(2) Study content and results
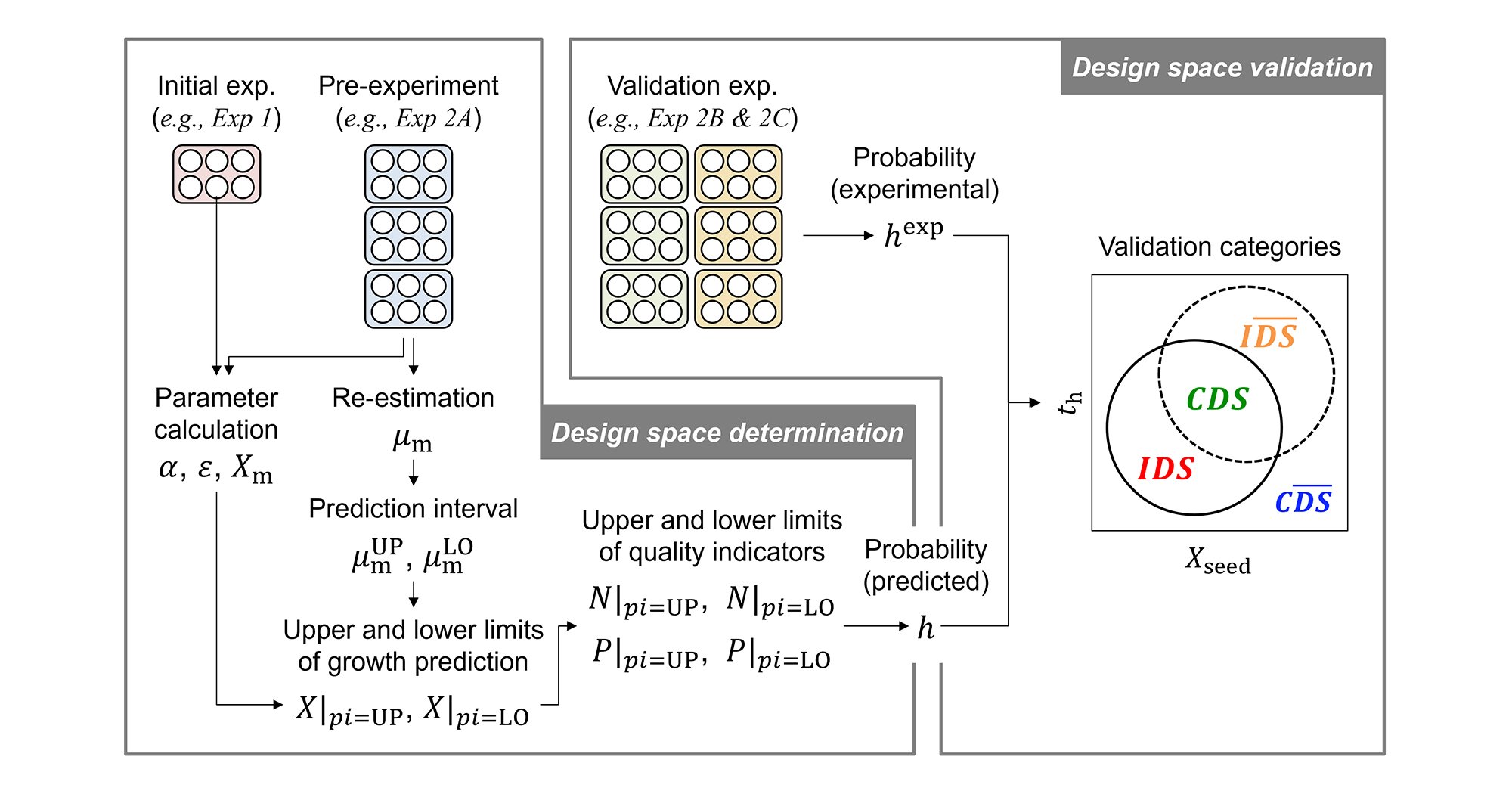
In this study, we developed a new mathematical model using the data obtained from automated experiments, which has enabled the identification of the design space using simulations and the optimization of cultivation conditions. Using an automated cultivating system with 12 parallel small-scale (250 mL) bioreactors, a cultivation experiment of CHO-MK cells under 29 conditions was performed. In the experiment, six design variables, such as agitation rate and dissolved oxygen (DO) concentration, were set to obtain sufficient experimental data for a wide range of cultivation conditions. Using these data, a mechanistic model*6 based on the mass balance and a data-driven model*7 that estimates the reaction rate constant in the mechanistic model were integrated to develop a hybrid model*8. When validation experiments were conducted under 15 conditions, high predictive accuracy was confirmed in 12 conditions, suggesting that this model is applicable to a wide range of conditions (Figure 2). Subsequently, the model was used to simulate six design variables, and as a result, a heatmap of the concentration of each component during cultivation (Figure 3), a design space that takes into account multiple impurities, and an optimal combination of design variables to maximize antibody production (Figure 4) could be obtained.

Figure 2 Experimental validation results of model predictive accuracy (Excerpt from [Nemoto, et al., 2026]; Used with permission)
As an example, the results of viable cell density are shown. Of the total 15 conditions, high predictive accuracy was confirmed in a wide range of cultivation conditions (12 conditions) except for the gray area (3 conditions) in the upper right corner.

Figure 3 Heatmap of the simulation results (Excerpt from [Nemoto, et al., 2026]; Used with permission)
In the heatmaps of viable cell density and mAb, lighter colors correspond to higher values, and in the other graphs, lighter colors correspond to lower values. The lighter the color, the more favorable the result. The white area in the upper right corner of the right figure was excluded from the simulation due to the low predictive accuracy of the model.

Figure 4 Optimal cultivation conditions to maximize design space and mAb concentration (Excerpt from [Nemoto, et al., 2026]; Used with permission)
The design spaces for dissolved oxygen concentration, agitation rate, and glucose feed rate are represented by the white area. The blue, green, and orange lines represent the constraint conditions of the impurities HCP, DNA, and acidic charge variants, respectively. The white area in the upper right corner of the right figure was excluded from the simulation due to the low predictive accuracy of the model.
(3) Future prospects
In this study, based on the data obtained from the automated cultivation experiments, we developed a new mathematical model that can accurately describe the concentrations from antibody to multiple impurities under a wide range of cultivation conditions, which has enabled the identification of optimal cultivation conditions using simulation. Going forward, we will integrate automated experiments and mathematical modeling further and develop research into the design of the entire process, including the subsequent purification step. Through this research, we aim to further advance digital technologies for design space construction and process optimization and contribute to further accelerating and efficient pharmaceutical process development.
Presenters and Researchers Information
Department of Chemical System Engineering, Graduate School of Engineering, The University of Tokyo:
Kosuke Nemoto (Ph.D Student)
Yuki Yoshiyama (Ph.D Student)
Yusuke Hayashi (Assistant Professor)
Sara Badr (Associate Professor)
Hirokazu Sugiyama (Professor)
Chitose Laboratory Corporation:
Mizuki Morisasa (Senior BioEngineer)
Junshin Iwabuchi (Principal BioEngineer)
Information About the Published Article
Journal name: AIChE Journal
Title: Hybrid modeling for optimizing cell cultivation in mAb production using a small-scale automated experimental platform
Authors: Kosuke Nemoto, Yuki Yoshiyama, Mizuki Morisasa, Junshin Iwabuchi, Yusuke Hayashi, Sara Badr, Hirokazu Sugiyama*
*Corresponding author
DOI: 10.1002/aic.70231
URL: https://doi.org/10.1002/aic.70231
Research Grant
This study was conducted with a grant from the Japan Agency for Medical Research and Development (AMED) (Project Focused on Developing Key Technology for Discovering and Manufacturing Drugs for Next-generation Treatment and Diagnosis “Development of Internationally Competitive Manufacturing Technology for Next-Generation Antibody Drugs: Digital platform for development and control of biopharmaceutical manufacturing processes; Project leader: Hirokazu Sugiyama).” This study was also supported by a grant from the Japan Science and Technology Agency (JST) (Support for Pioneering Research Initiated by the Next Generation: SPRING; JPMJSP2108).
Terminology Explanation
*1. Monoclonal antibody (mAb): The immune system of animals produces proteins called “antibodies” when they detect foreign substances (antigens) such as bacteria that have entered the body. mAbs are artificially produced antibodies that bind to only one specific type of marker (antigenic determinant or epitope) in an antigen. Antibody drugs with monoclonal antibodies as active ingredients are used to treat diseases such as cancer and Alzheimer’s disease using the antigen-antibody reaction.
*2. Design space: The multidimensional combination of input variables and process parameters that have been demonstrated to provide assurance of quality. This term is defined in the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q8 (R2) Pharmaceutical Development Guideline.
*3. Host cell proteins (HCPs): A general term for proteins derived from cells other than the target substance among process-related impurities in the manufacture of antibody drugs.
*4. Charge variants: This term refers to variants in which the charge of the mAb molecule differs from that of its original component (main component). There are those whose charge has shifted to the acidic or basic side and these two types of variants are called acidic charge variants and basic charge variants, respectively. These are classified as product-related impurities.
*5. Quality by Design (QbD): A systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management. This term is defined in ICH Q8 (R2) Pharmaceutical Development Guidelines.
*6. Mechanistic model: This term refers to a model described based on physical, chemical, and biological principles. This model has the advantage of being reliable and excellent extrapolation, as long as we have a good understanding of the phenomenon. It is also called the “fundamental model” or “white box model.”
*7. Data-driven model: This term refers to a model described solely based on the input-output relationships in the model. Even if we do not have a sufficient understanding of the mechanism of the phenomenon we aim to model, this model has the advantage of being easily built if we have enough high-quality data. It is also called the “statistical model” or “black box model.”
*8. Hybrid model: This model represents a combination of mechanistic model and data-driven model. It is used to enhance accuracy while maintaining interpretability. It is also called the “gray box model.”
About The University of Tokyo
The University of Tokyo is Japan's leading university and one of the world's top research universities. The vast research output of some 6,000 researchers is published in the world's top journals across the arts and sciences. Our vibrant student body of around 15,000 undergraduate and 15,000 graduate students includes over 5,000 international students.
https://www.u-tokyo.ac.jp/en/index.html
CHITOSE Group Overview
https://chitose-bio.com/
CHITOSE Group is a family of biotechnology companies leading the global bioeconomy. To live in abundance beyond the next millennium using the ability of living things, CHITOSE pursues the possibilities of biotechnology through technological and business development collaborating with its business partners all over the world.
About CHITOSE BIO EVOLUTION PTE. LTD. (head office that oversees CHITOSE Group)
・Established in October, 2011
・Head Office located in Singapore
・CEO: Tomohiro FUJITA, Ph. D.
About CHITOSE Laboratory Corp. (a core company of CHITOSE Group that focuses on business and technological development)
・Established in November, 2002
・Head Office located in Kawasaki-city, Kanagawa Prefecture, Japan
・CEO: Tomohiro FUJITA, Ph. D.
・COO: Rie KUGIMIYA
You May Also Like
These Related Stories
Hybrid Modeling of CHO Cell Cultivation in Monoclonal Antibody Production with an Impurity Generation Module