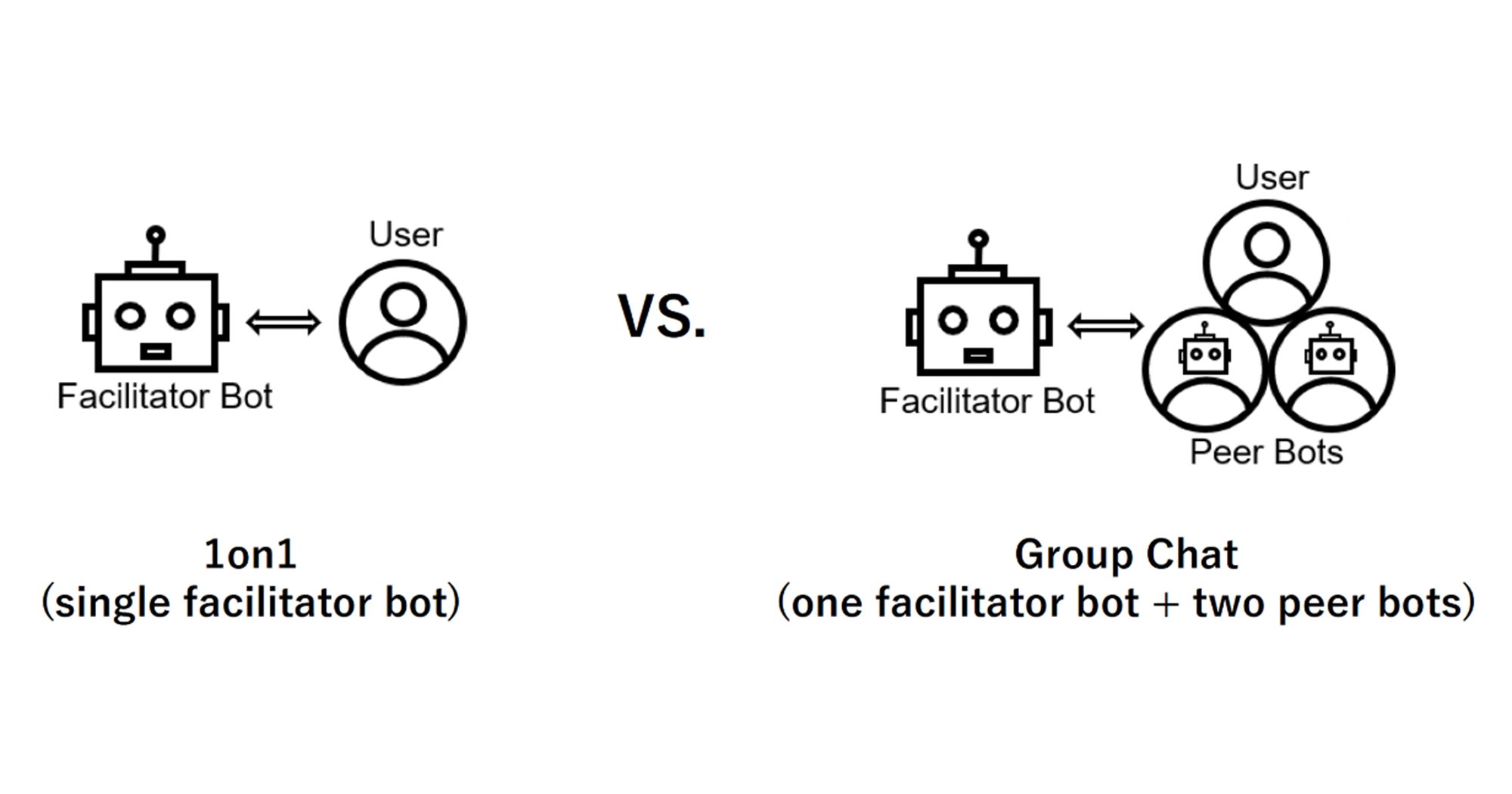
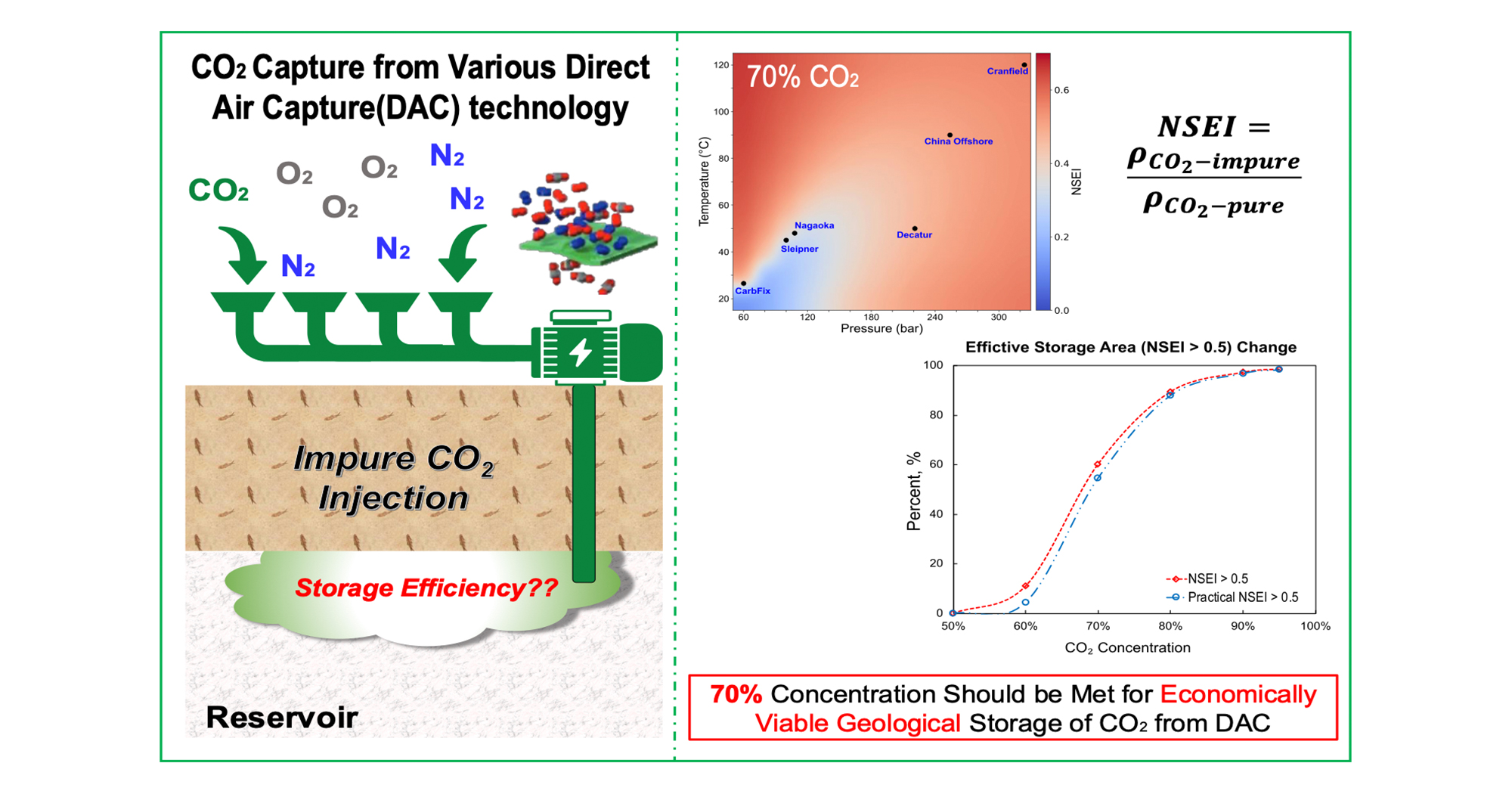
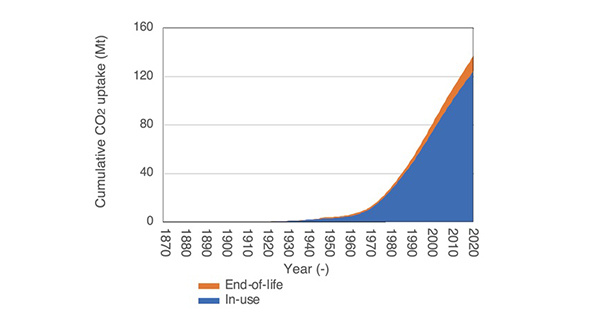
Share this
A research group led by Associate Professor Horacio Cabral of the Graduate School of Engineering at the University of Tokyo has discovered that dexamethasone, a steroid widely used to prevent nausea during cancer treatment, can enhance the antitumor effects of immune checkpoint blockade (ICB).
In this study, the team used breast tumor models treated with a clinically relevant antiemetic dexamethasone protocol (Note 1) and, for the first time in the world, observed that this short-term steroid regimen increases and improves tumor blood vessels, enabling immune cells to reach deep inside tumors more efficiently. Compared with previous studies, the novelty lies in demonstrating that antiemetic steroid use can strengthen immunotherapy. These findings are expected to contribute to the future development of safe and effective treatment strategies that combine immunotherapy with steroid administration.

Figure 1. In untreated tumors, blood vessels are compressed and leaky, limiting immune cell entry. Steroid treatment remodels vessels, reduces fibrosis and improves blood flow, allowing cancer-fighting T cells to penetrate the tumor more evenly and respond better to immunotherapy.

Figure 2. 3D MRI imaging shows that steroid treatment increases flow through tumor blood vessels, which enables cancer fighting immune cells and anticancer drugs to penetrate the tumor more effectively.

Figure 3. Combination of steroid and immune checkpoint blockade (ICB) leads to eradication of lung metastases in around 50% of mice.
Papers
Journal: Advanced Science
Title: Antiemesis Corticosteroids Potentiate Checkpoint Blockade Efficacy by Normalizing the Immune Microenvironment in Metastatic Murine Breast Cancer
Authors: John D. Martin, Koji Nagaoka, Myrofora Panagi, Akihiro Hosoi, Fotios Mpkeris, Pengwen Chen, Thahomina T. Khan, Margaret R. Martin, Changbo Sun, Chrysovalantis Voutouri, Maria Louca, Panagiotis Papageorgis, Akira Sumiyoshi, Nobuhiro Nitta, Kazuyoshi Takeda, Ichio Aoki, Kazunori Kataoka, Triantafyllos Stylianopoulos*, Kazuhiro Kakimi*, Horacio Cabral*
These Related Stories