PRESS RELEASE
- Research
- 2015
Advance on the development of novel dinuclear ruthenium and iron complexes as efficient organometallic anode catalysts for the fuel cell
Authors
Masahiro Yuki, Ken Sakata, Yoshifumi Hirao, Nobuaki Nonoyama, Kazunari Nakajima, and Yoshiaki Nishibayashi
Abstract
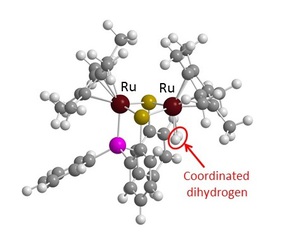
We have found that thiolate-bridged dinuclear ruthenium- and iron-complexes work as robust and efficient catalysts toward oxidation of molecular dihydrogen in protic solvent such as water and methanol under ambient reaction conditions. The catalytic activity of the complexes toward the chemical oxidation of molecular dihydrogen achieves up to ca. 10000 TON and electrooxidation of molecular dihydrogen proceeds quite rapidly. The high proton conductivity by the Grotthus mechanism in protic solvents seems to play a crucial role for the high performance of the catalysis. The experimental result and the density functional theory (DFT) calculation indicate that heterolytic cleavage of the coordinated molecular dihydrogen at the dinuclear complexes and the sequential oxidation of the hydride complexes are key steps to promote the present catalytic reaction. Synergistic effect between the two ruthenium atoms is observed during the catalytic transformation of molecular dihydrogen. We have confirmed the ability of the thiolate-bridged dinuclear ruthenium-complex as a good anode catalyst for the fuel cell although the present ability of the dinuclear ruthenium-complex as an anode catalyst is lower than that of Pt catalyst in the fuel cell. We believe that the thiolate-bridged dinuclear ruthenium-complex has the highest performance as organometallic fuel cell. It is noteworthy that the present dinuclear complex worked not only as an effective catalyst toward chemical and electrochemical oxidation of molecular dihydrogen but also as a good anode catalyst for the fuel cell. We consider that the result described in this paper provides useful and valuable information to develop highly efficient and low-cost transition metal complexes, instead of Pt, as anode catalysts in the fuel cell.
Contact information:
Yoshiaki Nishibayashi
Institute of Engineering Innovation, School of Engineering, The University of Tokyo, Yayoi, Bunkyo-ku, Tokyo 113-8656, Japan
Ken Sakata
Faculty of Pharmaceutical Sciences, Hoshi University, Ebara, Shinagawa-ku, Tokyo 142-8501, Japan
Yoshifumi Hirao
Fuel Cell System Development Center, Toyota Motor Corporation, Mishuku, Susono, Shizuoka 410-1193, Japan
For further information, please visit here.