PRESS RELEASE
- Research
- 2021
Molecular basis of glycyl-tRNAGly acetylation by TacT from Salmonella Typhimurium
Authors
Yuka Yashiro, Chuqiao Zhang, Yuriko Sakaguchi, Tsutomu Suzuki, Kozo Tomita
Summary
Bacterial toxin-antitoxin modules contribute to the stress adaptation, persistence, and dormancy of bacteria for survival under environmental stresses and are involved in bacterial pathogenesis. In Salmonella Typhimurium, the Gcn5-related N-acetyltransferase toxin TacT reportedly acetylates the α-amino groups of the aminoacyl moieties of several aminoacyl-tRNAs, inhibits protein synthesis, and promotes persister formation during the infection of macrophages. Here, we show that TacT exclusively acetylates Gly-tRNAGly in vivo and in vitro. The crystal structure of the TacT:acetyl-Gly-tRNAGly complex and the biochemical analysis reveal that TacT specifically recognizes the discriminator U73 and G71 in tRNAGly, a combination that is only found in tRNAGly isoacceptors, and discriminates tRNAGly from other tRNA species. Thus, TacT is a Gly-tRNAGly-specific acetyltransferase toxin. The molecular basis of the specific aminoacyl-tRNA acetylation by TacT provides advanced information for the design of drugs targeting Salmonella.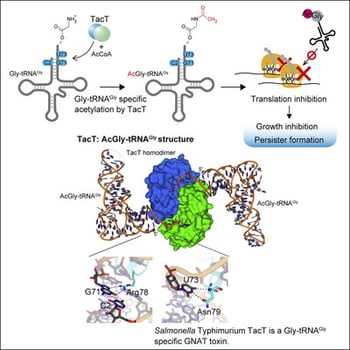
Science Direct : https://www.sciencedirect.com/science/article/pii/S2211124721016260?via%3Dihub

