PRESS RELEASE
- Research
- 2021
Emerging Disordered Layered-Herringbone Phase in Organic Semiconductors Unveiled by Electron Crystallography
Authors
Satoru Inoue, Kiyoshi Nikaido, Toshiki Higashino, Shunto Arai, Mutsuo Tanaka, Reiji Kumai, Seiji Tsuzuki, Sachio Horiuchi, Haruki Sugiyama, Yasutomo Segawa, Kiyofumi Takaba, Saori Maki-Yonekura, Koji Yonekura, Tatsuo Hasegawa
Abstract
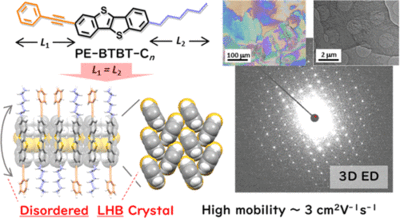
The control of two-dimensional layered crystalline and/or liquid crystalline phases for π-extended organic molecules is crucial for expanding the potential of organic electronic materials and devices. In this work, we develop unique solution-processable organic semiconductors based on the unsymmetric substitution of [1]benzothieno[3,2-b][1]benzothiophene (BTBT) with two different substituents, namely, phenylethynyl (PE) and normal alkyl with different chain lengths n (−CnH2n+1), both of which exhibit structural flexibility while maintaining the rod-like nature over the entire molecule. A distinctive layered solid crystalline phase, analogous to the smectic liquid crystalline phase, is obtainable in PE-BTBT-Cn at n = 6, where the substituent lengths are almost the same. The BTBT moiety maintains a rigid layered-herringbone (LHB) packing, whereas the molecular long axis exhibits a complete orientational disorder. We refer to this packing as disordered LHB (d-LHB), the unique geometry of which can be analyzed by the emerging technique of microcrystal electron diffraction crystallography. The intermolecular core–core interactions stabilize the d-LHB packing, enabling a relatively high field-effect mobility of approximately 3 cm2 V–1 s–1. In contrast, PE-BTBT-Cn with longer alkyl chains (n = 8, 10, 12) exhibits higher mobility of approximately 7 cm2 V–1 s–1 by constituting bilayer-type LHB (b-LHB), which is associated with the unsymmetrical length of the substituents. We discuss the correlation and competition among the d-LHB, b-LHB, and smectic liquid crystalline phases based on the structural, thermal, and transistor characteristics. These findings demonstrate the controllability of various phases in layered organic semiconductors.
Chemistry of Materials:https://pubs.acs.org/doi/10.1021/acs.chemmater.1c02793

