PRESS RELEASE
- Research
- 2014
A new battery utilizing a redox between oxide and peroxide -Innovative rechargeable battery beyond the current ones-
Authors
Shin-ichi Okuoka, Yoshiyuki Ogasawara, Yosuke Suga, Mitsuhiro Hibino, Tetsuichi Kudo, Hironobu Ono, Koji Yonehara, Yasutaka Sumida, Yuki Yamada, Atsuo Yamada, Masaharu Oshima, Eita Tochigi, Naoya Shibata, Yuichi Ikuhara, Noritaka Mizuno
Abstract
The demand for rechargeable batteries is increasing as power sources for not only portable electronic appliances but also large-scale application such as electronic vehicles and stationary energy system. The improvement of their performance is needed in terms of energy density, capacity, safety, and cost-effectiveness. Therefore, the development of innovative rechargeable batteries which will be highly superior to the current lithium ion battery system is desired.
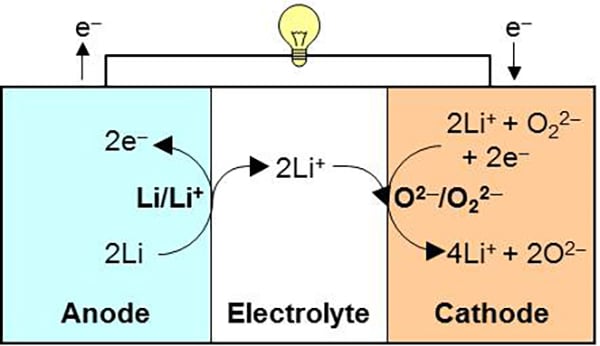
Professor Noritaka Mizuno at Department of Applied Chemistry, Graduate School of Engineering, the University of Tokyo and his research group collaborating with Nippon Shokubai Co., Ltd. have developed a new rechargeable battery system operating on a redox reaction between oxide and peroxide in cathode. Its theoretical specific energy is seven times as large as that of the current lithium ion battery based on the total weight of cathode and anode active materials. The group demonstrated that peroxide species were formed in the charge process and consumed in the discharge process with cobalt-doped lithium oxide cathode material.
This new rechargeable battery system can exhibit larger specific energy and capacity than the existing lithium-ion batteries. The battery system is expected to be a practical high-performance next-generation battery for electronic vehicles and stationary energy system.
This research is supported by the Japan Society for the Promotion of Science (JSPS) through its “Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program)”.