PRESS RELEASE
- Research
- 2016
Hydrate-melt electrolytes for high-energy-density aqueous batteries
Authors
Yuki Yamada, Kenji Usui, Keitaro Sodeyama, Seongjae Ko, Yoshitaka Tateyama & Atsuo YamadaAbstract
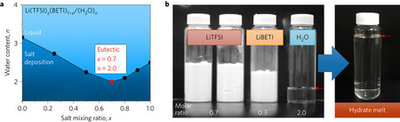
Aqueous Li-ion batteries are attracting increasing attention because they are potentially low in cost, safe and environmentally friendly. However, their low energy density (−1 based on total electrode weight), which results from the narrow operating potential window of water and the limited selection of suitable negative electrodes, is problematic for their future widespread application. Here, we explore optimized eutectic systems of several organic Li salts and show that a room-temperature hydrate melt of Li salts can be used as a stable aqueous electrolyte in which all water molecules participate in Li+ hydration shells while retaining fluidity. This hydrate-melt electrolyte enables a reversible reaction at a commercial Li4Ti5O12negative electrode with a low reaction potential (1.55 V versus Li+/Li) and a high capacity (175 mAh g−1). The resultant aqueous Li-ion batteries with high energy density (>130 Wh kg−1) and high voltage (∼2.3–3.1 V) represent significant progress towards performance comparable to that of commercial non-aqueous batteries (with energy densities of ∼150–400 Wh kg−1 and voltages of ∼2.4–3.8 V).
Nature Energy URL: http://www.nature.com/articles/nenergy2016129

