PRESS RELEASE
- Research
- 2022
Photoactivatable Materials for Versatile Single-Cell Patterning Based on the Photocaging of Cell-Anchoring Moieties through Lipid Self-Assembly
Authors
Shinya Yamahira, Ryuji Misawa, Takahiro Kosaka, Mondong Tan, Shin Izuta, Hayato Yamashita, Yuji Heike, Akimitsu Okamoto, Teruyuki Nagamune, Satoshi Yamaguchi
Abstract
Versatile methods for patterning multiple types of cells with single-cell resolution have become an increasingly important technology for cell analysis, cell-based device construction, and tissue engineering. Here, we present a photoactivatable material based on poly(ethylene glycol) (PEG)-lipids for patterning a variety of cells, regardless of their adhesion abilities. In this study, PEG-lipids bearing dual fatty acid chains were first shown to perfectly suppress cell anchoring on their coated substrate surfaces whereas those with single-chain lipids stably anchored cells through lipid–cell membrane interactions. From this finding, a PEG-lipid with one each of both normaland photocleavable fatty acid chains was synthesized as a material that could convert the chain number from two to one by exposure to light. On the photoconvertible PEG-lipid surface, cell anchoring was activated by light exposure. High-speed atomic force microscopy measurements revealed that this photocaging of the lipid–cell membrane interaction occurs because the hydrophobic dual chains self-assemble into nanoscale structures and cooperatively inhibit the anchoring. Light-induced dissociation of the lipid assembly achieved the light-guided fine patterning of multiple cells through local photoactivation of the anchoring interactions. Using this surface, human natural killer cells and leukemia cells could be positioned to interact one-by-one. The cytotoxic capacity of single immune cells was then monitored via microscopy, showing the proof-of-principle for applications in the high-throughput analysis of the heterogeneity in individual cell–cell communications. Thus, the substrate coated with our photoactivatable material can serve as a versatile platform for the accurate and rapid patterning of multiple-element cells for intercellular communication-based diagnostics.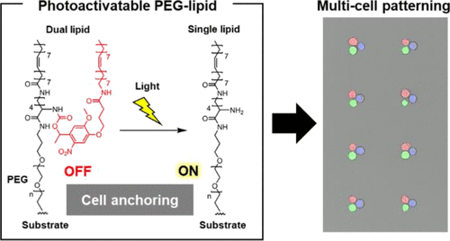
Journal of the American Chemical Society: https://pubs.acs.org/doi/10.1021/jacs.2c02949

